Study Description
The National Myelodysplastic Syndrome Study (MDS) is a multi-center, prospective cohort study enrolling patients from centers in the National Cancer Institute (NCI) National Clinical Trials Network (NCTN) and NCI Community Oncology Research Program (NCORP). The first study site was activated on May 17, 2016, with enrollments officially beginning on June 13, 2016. The study is now actively recruiting new patients with a targeted accrual of 2000 participants of MDS or MDS/MPN overlap disorders. The enrollment period is estimated to close on April 30, 2024. The study’s ClinicalTrials.gov identifier is NCT02775383.
The goal of the National MDS Study is to establish a publicly available resource to facilitate the study of MDS natural history. This will be accomplished through: 1) Creation of a multi-institutional, longitudinal collection consistently processed and clinically well-annotated blood and tissue specimens collected prospectively from participants with MDS and participants with idiopathic cytopenia of undetermined significance (ICUS), and 2) Support for investigator-initiated studies of MDS that will have high-impact for MDS patients, including basic science, clinical, health outcomes and epidemiological research.
Please review the design and methodology manuscript (Sekeres et. al., 2019) that was published in Leukemia & Lymphoma. Click here to access the online publication.
Study Design
The study has two main cohorts. The Longitudinal Cohort contains participants with MDS, MDS/MPN overlap disorder, AML <30% blasts without core binding factor or acute promyelocytic leukemia, ICUS, or at-risk based on a diagnosis of dysplasia or select karyotypic or genetic abnormalities. All of these patients have a baseline and follow-up visits every 6-months. The Cross-sectional Cohort contains participants who are not diagnosed with MDS, MDS/MPN overlap disorder, or ICUS and have only a baseline visit.
Please refer to the following schematic detailing the MDS study workflow.
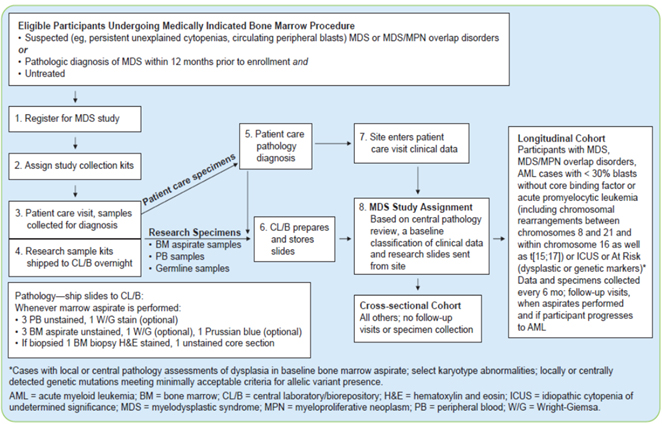
Overview of Study Procedures
Informed consent is the first step in the enrollment process for all eligible participants. Study specific procedures begin once consent is provided for collection of data and specimens. Participant histories are then collected, and a medical chart review is performed to obtain past medical history, baseline laboratory test results, and diagnostic information including reports and treatment history. Participants are asked to provide samples including eyebrow hair follicles and a buccal swab. Peripheral blood and bone marrow samples are also collected for long-term storage. Slides are created by the clinical site from bone marrow, peripheral blood, and core biopsy to support a central review by study hematopathologists.
Based on the central review, patients are later classified into the longitudinal cohort (includes the following conditions: MDS, MDS/MPN overlap disorders, AML with <30% blasts without core binding factor or APL, or ICUS) or into the cross-sectional cohort (all other cases). Patients in the longitudinal cohort are followed every 6 months while those in the cross-sectional cohort are not followed. Sample collection is terminated at any point during the study where the participant receives a hematopoietic cell transplantation (HCT). A final sample is also expected to be collected from longitudinal cases at the time of any AML diagnosis. No additional follow-up samples are expected for AML cases with >30% blasts, core biding factor, or acute promyelocytic leukemia.
Participants who are classified as at-risk for developing MDS are also followed as part of the Longitudinal Cohort. The algorithm for determining a participant’s at-risk status is defined as follows. If dysplasia or karyotype abnormalities are detected, the participant is assigned to the “At-Risk” cohort. The participant’s assignment, however, remains pending if neither of these two criteria are met until results of a central targeted sequencing panel are received. If central genetic results, or any local genetic results provided, identify mutations in any of the 53 protocol defined genes of interest, the case is then assigned to the “At-Risk” cohort and followed longitudinally, otherwise they are assigned to the Cross-sectional Cohort.
Patients in the longitudinal cohort are expected to complete a study visit every 6 months within a +/- 60 day window. If a participant’s cohort assignment is pending the outcome of the targeted sequencing panel at 6 months, the site proceeds with standard data and sample collection. The participant is automatically assigned to the cross-sectional cohort if they do not receive an assignment at 12 months.
Please reference the NHLBI-MDS Protocol section 5.2.1.1 for a list of data collected at baseline and section 5.2.2 for the procedures at Follow-up.
Quality of Life questionnaires are collected at baseline for all participants, and 6 months, 12 months, and then yearly thereafter for Longitudinal cases. After baseline, participants diagnosed with MDS, MDS/MPN and AML (<30 % blasts) complete the Quality of Life in Myelodysplasia Scale (QUALMS), Functional Assessment of Cancer Therapy – General (FACT-G Version 4) and Patient Reported Outcomes Measurement Information Systems (PROMIS) Short Form v1.0 Fatigue 7a, and ED-5D-5L. Participants in the ICUS and At-Risk cohorts after baseline complete only the PROMIS Short Form v1.0 – Fatigue 7a and EQ-5D-5L.
Biologic Samples
Biological materials are collected from participants according to the following table and submitted to the Central Lab and Biorepository (CL/B) for processing and storage:
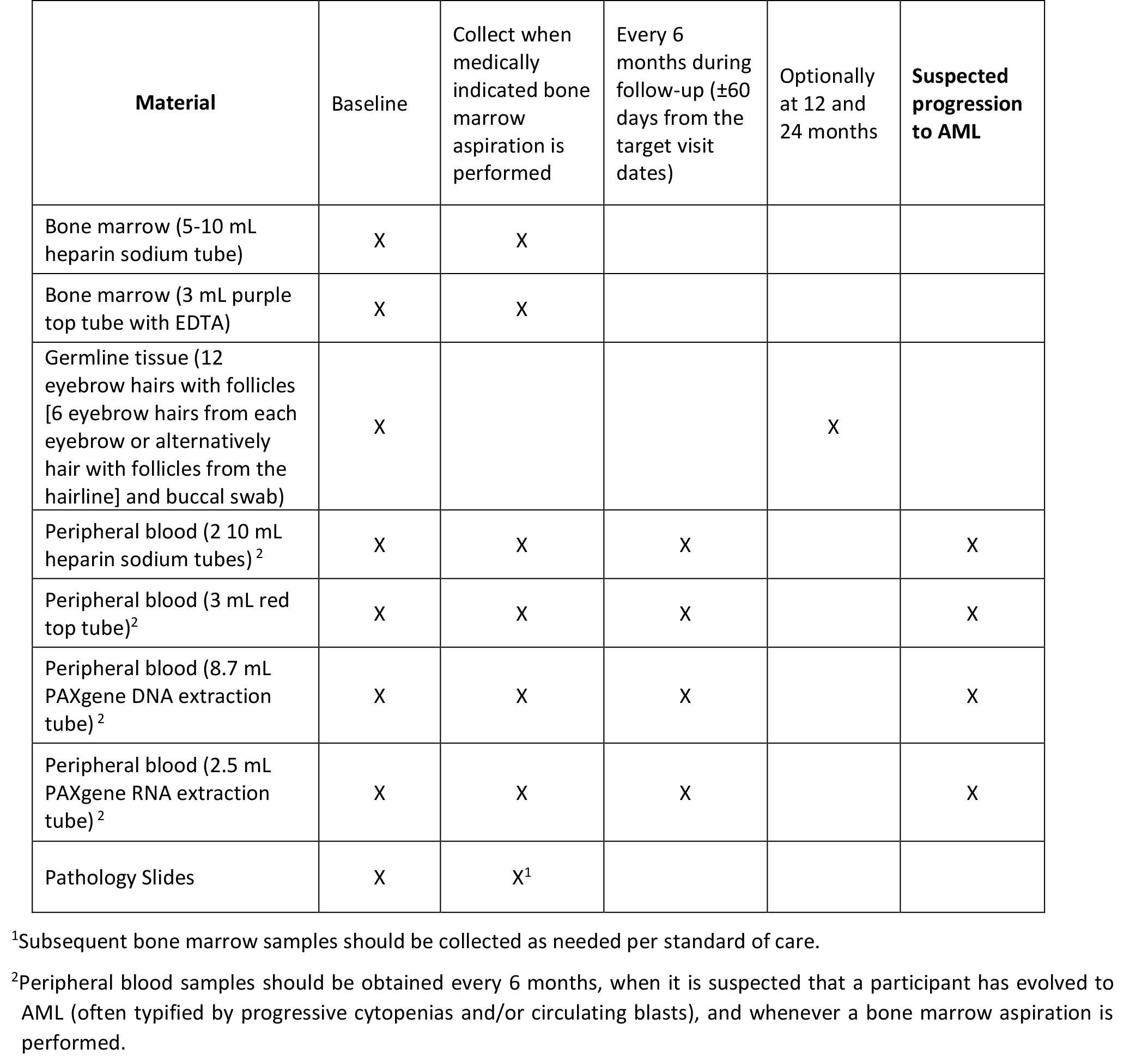
Pathology materials, including bone marrow aspirate and pathology slides at enrollment, are required to be submitted by the clinical sites to support diagnostic review for participant diagnosis and cohort assignment. Unstained peripheral blood and bone marrow aspirate slides are required in addition to an H&E-stained slide of the bone marrow biopsy, if a biopsy procedure was performed. A touch preparation slide is also required if a dry tap occurs. The pathology materials are shipped within 24 hours of collection to the CL/B.
All specimens shipped to the CL/B, as noted above, are linked to participants using a study barcode label and upon receipt at the CL/B are processed and stored at -80 degrees Celsius. Frozen cells are stored in liquid nitrogen. Ultimately at the end of the study all specimens and their associated data will be transferred to the NHLBI Biorepository for long term storage and made available for future research.
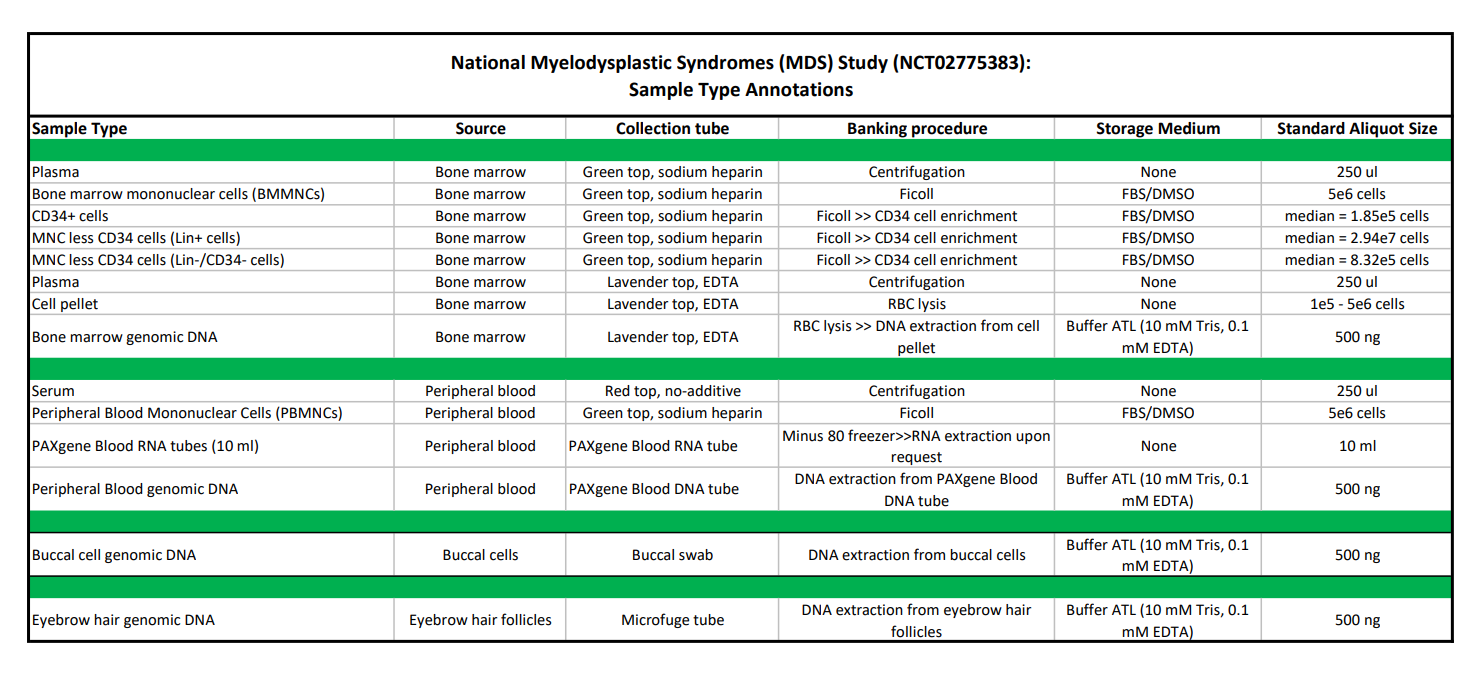
Details on the collection tubes, processing procedures, storage medium, and standard aliquot sizes for each of the biospecimens available for request are defined in the table below. Please click here to download a PDF version of the table.
Eligibility Criteria
Patients are eligible to join the study if they have a suspected MDS/MPN overlap disorder and are undergoing diagnostic workup and bone marrow assessments, or if they have been diagnosed with MDS within 6-months of enrollment and undergoing clinical evaluation and bone marrow assessments to confirm MDS or evaluate disease status. Enrolling sites for this study include those that are members of the NCTN or the NCORP. For detailed eligibility criteria please reference NHLBI-MDS Protocol section 3.1.