Request Workflow Guide
Steps for requesting MDS resources
All requests for MDS resources are managed by the MDS Data Coordinating Center and should be submitted through the National MDS Natural History Study website, https://thenationalmdsstudy.net/. The process for requesting data and/or specimens involves a two-stage review (a feasibility assessment and a research proposal stage). The specific steps involved in each of the stages are defined below.
RESEARCH FEASIBILITY PHASE
First step: Developing a research hypothesis or question
Investigators are encouraged to review all posted materials on the public website concerning the MDS study design and protocol, case report forms, and the current cohort composition to help develop your research questions.
Second step: Querying the MDS Inventory Browser
Study feasibility can be assessed by using the MDS Inventory Browser, a web-based query tool, which can assist in defining a study cohort and identifies what resources are available for request. *To access the Inventory Browser a Registration Form must be submitted and approved. Once access is granted an email will be sent with initial login instructions.
A data dictionary defining each of the filter criteria is available for review in the browser in addition to a Browser User’ Guide to help guide one through the process of submitting a query. Available resources for request include clinical data such as diagnostic, laboratory and genetic testing results as well as digital bone marrow slide images (H&E and Wright stain) which are stored in the DCC’s AWS cloud storage area. Study biospecimens are also available including DNA, RNA, plasma, serum, MNCs, and cell pellets. The browser does not provide study outcome data rather it reports the number of patients in each diagnostic category that meet selected criteria. Please note data in the browser is only updated on an annual basis to correspond with the OSMB report data freeze.
The Inventory Browser generates a unique ID each time a user submits a query. This ID allows the system to maintain a history of the specific criteria selected so that the DCC can review and verify all results that are obtained by investigators. Please reference any pivotal query IDs in the electronic Research Feasibility Form to support our review of your application.
Third step: Submitting a Research Feasibility Form
An online Research Feasibility Form must be completed to begin the data/specimen request process for MDS resources. As noted above, please reference any query IDs that were used to generate browser results in the designated fields on the form. Investigators are expected to also provide the following data elements:
- Contact information
- Research details:
- Title of Proposed Research
- Description of Research
- Study population criteria:
- Diagnostic and risk category
- Demographics (race, gender, age, disposition)
- Data/Outcomes requested:
- Phenotypic/Clinical data
- Diagnostic information
- Demographics
- Disposition/Study Endpoints
- Medical History
- Quality of Life
- Laboratory data
- Laboratory assessments
- Cytogenetics
- Bone marrow assessments
- Bone marrow slide images
- Baseline genetic data
- Variant data
- Raw BAM files
- Phenotypic/Clinical data
- Other characteristics or outcomes
- Requested Specimens
- Specimen source
- Specimen type
- Timepoint
Upon submission of the electronic Research Feasibility Form, the DCC will begin its internal review and contact the investigator if there are any questions or if there is a need to clarify aspects of the application. If significant updates are needed to the request, the DCC may ask the investigator to submit a new Feasibility Form before it is circulated to the larger MDS Study Leadership Team for formal review.
Fourth Step: Feasibility Assessment Process
An assessment of each Feasibility form is performed by members of the MDS Study Leadership Team within approximately 14 days of submission. The review includes an evaluation of the proposed research to confirm availability of requested data in the central database and/or specimens in the central repository's inventory. The outcome of the evaluation will result in the proposal being deemed “feasible,” “not currently feasible, but may be in the future,” or “not feasible.” In certain cases, data and/or specimens in the MDS repository may not be sufficient to support the proposed research, however, the project may still be approved if the application can reference other available external sources of data and/or specimens which could be leveraged to improve the study's overall power. A proposal that is deemed feasible will open the next stage in the request process, while a proposal that was considered not feasible will be closed and the investigator will need to submit a new form if they would like to request additional resources.
Fifth Step: Feasibility Decision Notice and Invitation to Next Phase
All decisions regarding the feasibility assessment will be communicated to the applicant via email. If the research is approved, then the email will also include an invitation to complete a full research proposal. The current Research Proposal form will be attached in an email along with a Data and Materials Distribution Agreement (DMDA).
The DMDA is a contractual document used to govern the transfer of confidential or protected data and/or specimens generated directly by the MDS Study or by ancillary researchers. The document sets forth the limitations on use of the data, expected safeguards of the data, liability for harm arising from the use of the data, and privacy rights that are associated with the data transfer. Publication policies and rules regarding how to acknowledge the study in abstracts and publications are also outlined in the DMDA. The agreement must be signed by the Principal Investigator as defined in the Research Proposal Application as well as by an authorized official who has signatory authority at the PI’s institution. While data and specimen release by the DCC to an investigator is conditional upon the DMDA being signed, the agreement does not need to be put into place until after the proposal has been approved by NHLBI.
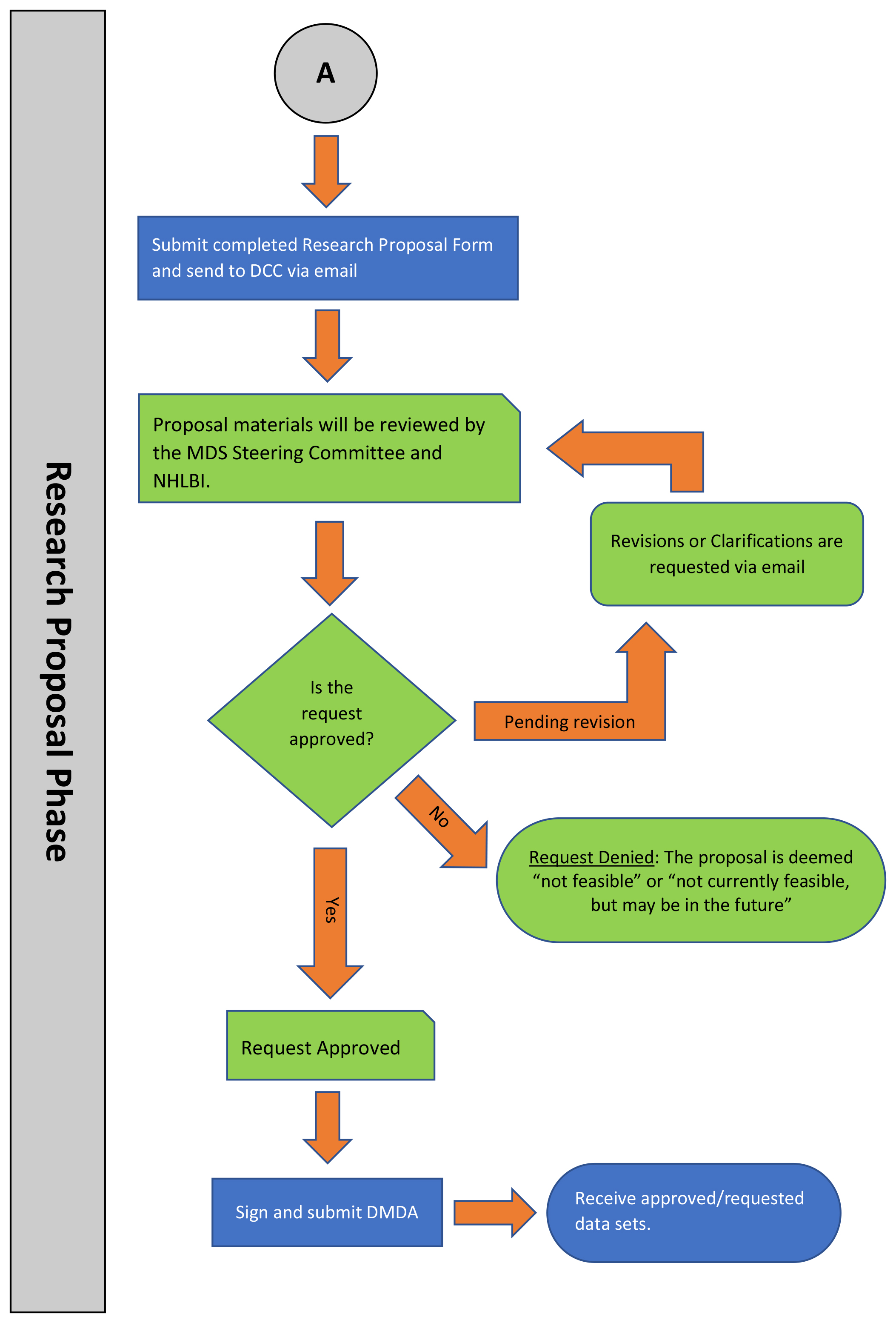
RESEARCH PROPOSAL PHASE
Sixth Step: Submitting a Research Proposal Form
The Research Proposal Form is designed similar to a grant application where hypotheses and detailed methods must be presented for review by a review committee. The form must be submitted back to the DCC within 6 months of the approved Research Feasibility Form or the request will be automatically closed. Closed requests may only be reopened through the submission of a new Research Feasibility Form. All submitted proposals are reviewed at least annually by the MDS Steering Committee and must be submitted at least 1 month prior to the committee’s annual meeting date.
The Research Proposal Form collects the following information:
- Contact Information
- A description of the institutional contribution of the request to the MDS Study [if applicable]
- Funding source for the proposed research [preference going to research already funded, is this for a grant]
- Indication of whether a letter of support is needed
- Research plan aims and hypotheses
- A detailed research plan including study design, eligibility criteria for selected study cohorts, variables to be used, statistical methods, and strengths/limitations of the research.
- A description of the significant/innovation of the proposed research
- Indication of whether an IRB review and approval is required
- Planned duration of the proposed research
- Planned conferences or journals for presentation of research results
- Services requested [evaluate this criteria and maybe remove]
The DCC will confirm receipt of the Research Proposal Form via email within approximately 14 days of receipt and correspond directly with the investigator if there are any questions and to confirm a timeline for the formal Steering Committee review.
Seventh Step: Research Proposal Review Process
The details of the Research Proposal Form are reviewed by the MDS Study Steering Committee during the annual meeting. The Steering Committee’s final recommendation on the resource request is forwarded to NHLBI who has final decision to approve or decline the request. The Steering Committee’s recommendation is based on their evaluation of the following review criteria:
- Are the proposed aims/hypotheses thoroughly described?
- Does the analysis plan contain sufficient details to specify how the data will be used and analyzed?
- Does the research represent a significant innovative contribution to the field?
Based on group consensus on how the proposal meets the above criteria, the committee will recommend either that the proposal be approved, revised, or rejected.
Eighth Step: Research Approval Decision
The details of the Steering Committee’s proposal review and the final approval decision from NHLBI will be sent to the investigator via email. An approved proposal will move to the next stage of the data/specimen release process, while a rejected request will automatically be closed. If the committee requests revisions, the investigator must address all concerns raised and resubmit the Research Request Proposal Form to the DCC. A revised proposal may be reviewed by the committee outside of the annual meetings via email depending upon the extent of the revisions and/or complexity of the overall request.
Nineth Step: Signing of the MDS DMDA
A fully executed DMDA are required of the approved investigator prior to the transfer of any generated study data sets and/or specimens. Please recognize that the process of obtaining institutional approval at this step may extend the timeline for delivery of data sets and therefore we recommend requestors identify the individual who is authorized to sign early in the research proposal process.
Tenth Step: Provision of Approved Analysis Datasets
The DCC will generate and provide de-identified individual-level analysis datasets to investigators with an approved research proposal and a signed DMDA. The timeline for the data transfer will be dependent on the complexity of the data sets being requested. Investigators can indicate a preference for the file format of the data (e.g., SAS, excel, csv). Files will be transferred securely using the DCC’s Box account. Other transfer methods such as providing a tokenized download link may be considered if the volume of data requires an alternate approach to Box.